| |
Ecology
Habitat.—Chelodina burrungandjii occupies permanent water ranging from pools in rocky sandstone gorges and at the base of the escarpment, to more open riverine and billabong habitats with fringing riparian vegetation and often dense submergent and emergent aquatic vegetation (e.g. Nymphea spp. Nymphoides spp.). In the Katherine River, it occurs in micro-sympatry with Emydura subglobosa worrelli (Emydura sp. aff. subglobosa of Georges and Adams, 1996) and in the Alligator Rivers region it occurs sympatrically with the common sawshell Elseya latisternum. More rarely, it can be found with Elseya dentata in plunge pools below the escarpment.
Reproductive cycles.—The ovaries of six mature females (all examined in October-November) were in early stages of vitellogenesis, typically containing small numbers (2-5) of enlarged vitellogenic follicles (3-9 mm in diameter) and larger numbers of smaller follicles (< 2 mm). Two of these females had small (< 1-2 mm diameter) corpora lutea on their ovaries, most likely remaining from the previous breeding season. The ovaries of two females were in a quiescent phase with no enlarged follicles, but the presence of ovarian scars indicated a history of breeding. The remaining female was in a late stage of regression with several small (< 3 mm) atretic follicles, identified as such by their discolouration (Georges, 1983), and small (< 1 mm diameter) corpora lutea.
Six mature males (all examined in October-November) had enlarged vascularised testes and distended, white epididymes indicating that sperimiogenesis and spermiation were underway. This pattern is broadly similar to that of C. rugosa in which testes and epididymes become enlarged in October-November and peak in January, followed by spermiation through February and March as testes and epididymes regress. Mating in C. rugosa is presumed to commence in January-February and may continue for several months (Kennett, 1994; 1999). More samples are needed to confirm if this pattern is typical of C. burrungandjii.
Diet.—Chelodina burrungandjii are predominantly carnivorous, feeding mainly on fish and shrimp. Nine turtles (39% excluding 7 with empty stomachs) had fed on fish, and fish accounted for 31% by weight of the pooled stomach contents. Fish species included the Purple-Spotted Gudgeon (Mogurnda mogurnda) but unidentified species were also present. Chelodina burrungandjii feeds voraciously in captivity and will devour 10-15 fish in a few minutes. Sixteen turtles (70% excluding 7 with empty stomachs) had fed on Macrobrachium shrimp. Shrimp comprised 49% by weight of the total diet. Legler (1982) also reports a predominance of shrimp and fish in the diet and also recorded Atyid shrimp, Orthoptera and crab (probably Holothusiana).
Chelodina burrungandjii also feeds on plant material. Five individuals (22% excluding 7 with empty stomachs) contained vegetation in their stomachs and vegetation comprised 20% by weight of the pooled stomach contents. In all but one case, vegetative matter comprised the entire contents. One individual had consumed 6.3 g of plant material including leaves from a Freshwater Mangrove (Barrangtonia acutangula) and bark, double the average weight of stomach contents (3.0 + 0.52g, n = 23). In one sample, the leaf material was folded and glued together presumably as shelter for an aquatic invertebrate and the leaf may have been ingested along with the invertebrate as the intended prey. Two individuals each contained an unidentified seed capsule. Faeces from an individual from the Mann River comprised leaves only.
|
|
Chelodina burrungandjii probably utilises both a sit-and-wait ambush strategy in addition to more active pursuit of prey. The broad flattened head likely represents an adaptation to a gape and suck mode of feeding (Pritchard, 1988; Legler and Georges, 1993). Sample sizes were inadequate for analysis but there did not appear to be sex or size bias in diet composition except that larger individuals (usually females) tended to consume larger individual prey items.
Discussion
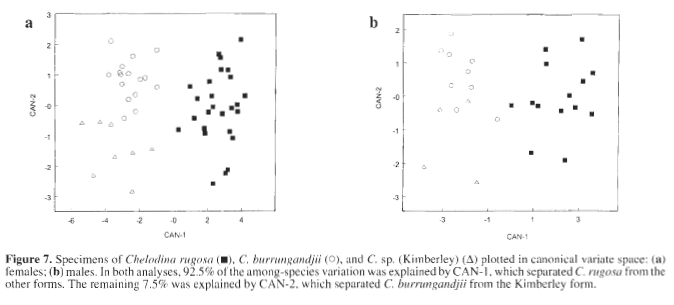
Chelodina burrungandjii is clearly a distinct species from C. rugosa, to which it was previously assigned. This is evident from the presence of discrete characters that diagnose the two and from the discriminant analysis based on measurements of the head and shell presented in this paper. It confirms the diagnostic differences identified using allozyme electrophoresis in a pilot study based on a single specimen of C. burrungandjii (Georges and Adams, 1992) and in a more substantial survey soon to appear (Georges et al., in prep). Other workers are currently preparing formal description of the Kimberley form so we do not present such a description here. It is clearly very similar to C. burrungandjii, much more so than either are to C. rugosa (Table 3). However, specimens can be reliably assigned to either C. burrungandjii or the Kimberley form on both discrete character states and females can be unambiguously assigned in the canonical discriminant space. Unpublished electrophoretic analyses of Georges et al. (in prep) indicates that the two have very recently diverged, lacking even a single fixed allelic difference.
The ecological data and anecdotal information provide additional evidence of a difference between C. burrungandjii and C. rugosa. The observed absence of large atretic follicles and large corpora lutea indicates that Chelodina burrungandjii had not nested in at least the 8-10 weeks prior to examination, but it is likely that they would have degenerated further than observed if they had nested at the end of the wet season in March/April as does C. rugosa (Kennett, 1994, 1999). Degeneration of corpora lutea on the ovaries of Chelodina rugosa is largely complete by the end of August and are only rarely observed later than this (Kennett 1994, 1999). However, we cannot be definitive on the distinction between the nesting seasons of the two species because in some years when conditions permit, C. rugosa may continue nesting into the dry season, as late as July/August (Kennett, 1994, 1999). Dry-season nesting of C. burrungandjii is consistent with the knowledge of local Aboriginal people who report that they find nests of C. burrungandjii during the dry season and that it digs nests in riverside sand banks like a freshwater crocodile (Phyllis Windjarra pers. comm., Sarah Flora, pers. comm.). This suggests that C. burrungandjii does not nest underwater like its congener C. rugosa (Kennett et al., 1992), but given the close taxonomic relationship between the two species (Georges and Adams, 1992), the tolerance of C. burrungandjii eggs to immersion (Kennett et al., 1993, 1998; Seymour et al., 1997) is worthy of further investigation.
|
|